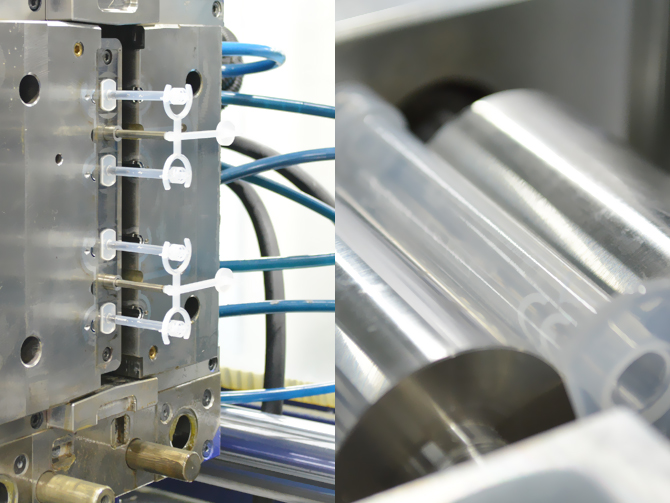
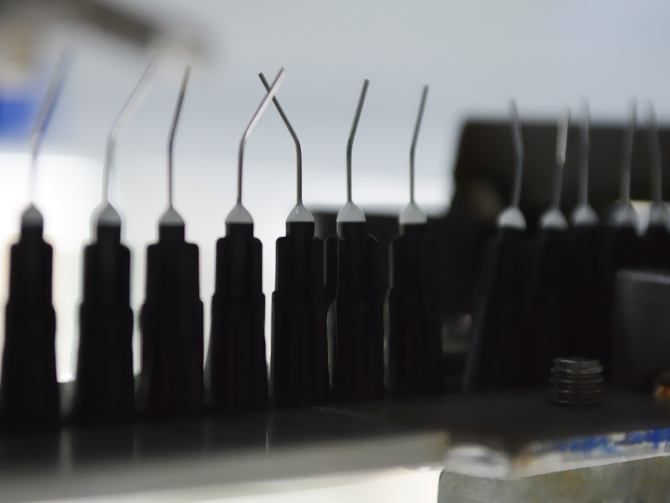
Complex tasks are mastered at Amedcon through our Project Management team. For this purpose, we form specialist teams that to plan, organize and process projects with our customers promptly and reliably.
Our project managers are responsible for all project steps from the creation of the functional requirements through the development of prototypes to mass production and are also responsible for the observance of scheduling and budget specifications over the entire period of the project. They visualize the course of the project in detailed project plans and are informed of the status of the project at all times. Problems occurring are in this way detected at an early stage and countermeasures are developed.
We always start with a discussions about a customer’s target and general description of the product. In this first step to share initial specifications, drawings, or engineering files regarding the projects so that we can work to meet the design specifications. We are always more than willing to sign non-disclosure agreements to protect customers’ technology, designs, or other intellectual property.
The quality requirements for medical device increase continuously. With our quality management we rely consistently on "Quality by Design". With this concept, quality is already systematically anchored in the products and processes by our quality and engineering team during the development phases. The Quality Assurance department works together closely with all other involved departments during all phases of the product origin process. Quality by Design is in this way not only the securest, but also the most economical way to bring medical device into the market.
Our project manager will work with all departments of product development under one roof: Program Management, Product and process development, Mold making, Automation Engineering, Product and process development, Quality Planning and Small Batch Production. In this way, we promote the intensive cooperation between Quality Assurance and the other departments to enable gap-free quality management through the entire product origin process, from the idea to the series production-ready product. In the process, we consistently rely on an integrated Quality by Design concept that reduces total costs, shortens the time to market, minimizes coordination work and reduces the risks of the product.

Risk management is established at the beginning of the product develop-ment stage
Identify, analyze and evaluate risks that could originate from a product or component
Evaluate the probability of occurrence, the frequency of discovery, and the severity of the risk
Formulate reduction measures for controlling risks.